
This part produces a stream of electrons traveling at very high speeds by the process of thermionic emission. This curved the path for large-scale manufacture and use of CRT TVs until the recent development of Liquid Crystal Display, Light Emitting Diode and Plasma TVs. The first commercial cathode ray tube television manufacture dates back to 1934 by the company Telefunken in Germany. It uses thermionic emissions in vacuum tubes to release electrons from a target. This type of cathode consists of a thin filament heated to a very high temperature by passing electric current through it. Johnson and Harry Weiner Weinhart of Western Electric. However, a hot cathode came into existence after being developed by John b. Earlier cathode ray tubes used cold cathodes. In 1907, cathode ray tube was first used in television when Russian scientist Boris Rosing passed a video signal through it to obtain geometric shapes on the screen. Braun is also credited with the invention of the cathode ray tube oscilloscope, also known as Braun’s Electrometer. Thomson, which employed only electrostatic deflection using two internal plates. The cathode beam was deflected by a magnetic field only, in contrast to the discharge tube used earlier in the same year by J.J. He used a phosphor-coated mica screen and a diaphragm to produce a visible dot. The earliest version of the cathode ray tube, Braun Tube, was invented in 1897 by the German physicist Ferdinand Braun. Thomson’s experiments with cathode rays led to the discovery of the electron, the first subatomic particle to be discovered. In the year 1897, the English physicist J.J. Arthur Schuster and William Crooks proved that cathode rays are deflected by electric and magnetic fields, respectively. Crookes tubes are partially vacuum tubes having two electrodes kept at a high potential difference to discharge cathode rays, from the negatively charged electrode, cathode.

This showed that cathode rays consist of negatively charged particles.The eminent physicist, Johann Hittorf discovered cathode rays in 1869 in Crookes tubes. He observed that cathode rays were deflected towards the positive plate of the electric field. Thomson applied an electric field in the path of cathode rays in the discharge tube. DISCOVERY OF ELECTRONS – THOMSON EXPERIMENT AND RESULT He discovered that cathode rays consist of negatively charged subatomic particles (now called electrons), present in all atoms of the elements. J Thomson discovered electrons while studying characteristics of cathode rays. Although, he could not establish what electric current or cathode rays comprise and what is moving from the negative terminal to the positive terminal.įinally, in 1897 J. William observed that when he applies a high electric potential across the discharge tube, then current flows from the negative terminal to the positive terminal in the form of rays. William Crooke, the British Scientist, in the year 1875 successfully created a near to perfect vacuum (0.01 mm of mercury) in a glass tube sealed at both ends with metal plates. William Crook is the first person to pass an electric current through a vacuum However, most of them failed as none of them were able to create a perfect vacuum.
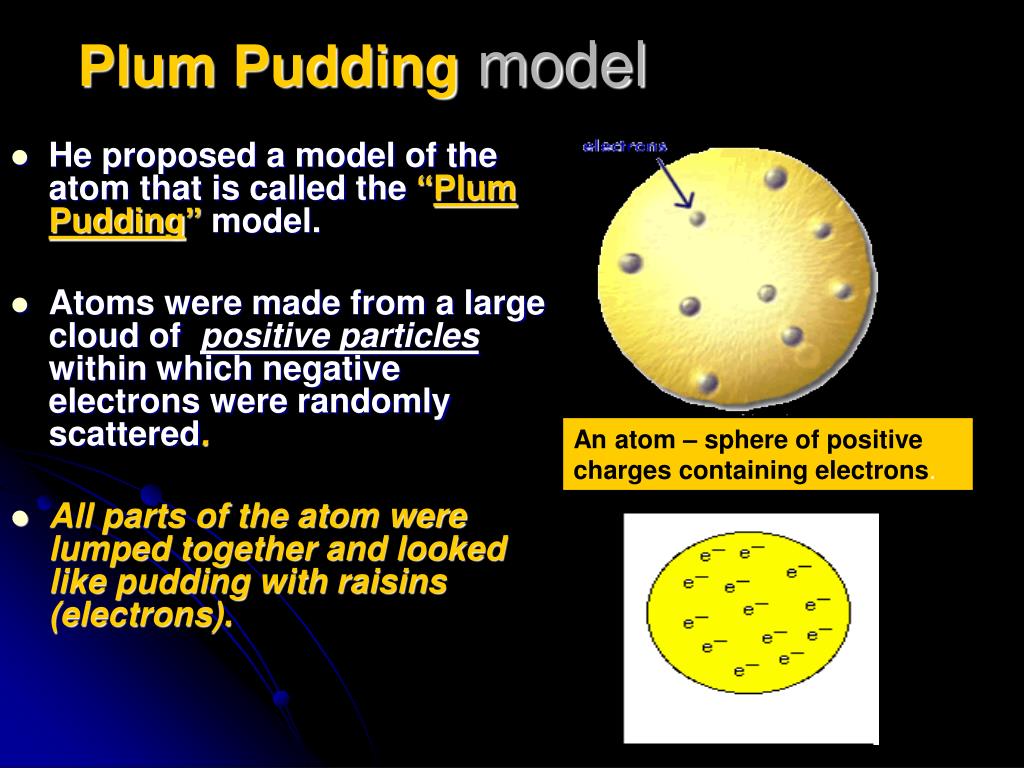
Cathode ray experiment for kids series#
After carrying out a series of experiments with – solids, liquids, and gases, scientists took one step further to drive electricity through a vacuum. They proved that the electricity or the electric current can flow through any substance – solid, liquid or gas if enough driving force or electric potential exists. The magnitude of the electric current flowing through a substance is directly proportional to the electric potential applied across it. The electric potential is a driving force that results in the flow of current through a substance due to the difference in concentration of charges at two ends of it. Scientists in the early nineteenth century were aware of electricity and the effect of electric potential. Who discovered electrons CATHODE RAY EXPERIMENT To know about the basic structure of an atom, click here This led to the discovery of the nucleus and other subatomic particles – protons and neutrons. Thomson discovered electrons, the race began among other scientists to uncover the basic structure of an atom. In his quest to study properties of cathode rays, he discovered that atoms contain negatively charged subatomic particles – ‘electrons’. But things changed after the discovery made by an English scientist named J.J. Thomson, the one who discovered electronsįor a long period in history, scientists were of opinion that atoms could not be broken further.


 0 kommentar(er)
0 kommentar(er)
